How to Find Work Function Given Wavelength
A small plate of a metal work function 117 eV is placed at a distance of 2 m from a monochromatic light source of wavelength 48 x 10-7 m and power 10 watt. The wavelength is inversely proportional to the frequency.

How To Convert Wavelength To Energy Youtube
We must convert nanometers into meters so this threshold wavelength value is 3 0 0 3 0 0 1 0 n m m.
. Any electromagnetic waves frequency multiplied by its wavelength equals the speed of light. λ λ 2. In that case the threshold.
Frequency should be 110e15 Hz. Subtract the KE of the electron from that to get the work function. Wavelength Velocity Frequency.
Stopping potential V s 263 V work function Φ 4 eV 4 x 16 x 10-19 J 64 x 10-19 J speed of light c 3 x 10 8 ms Plancks constant h 663 x 10-34 Js Charge on electron e 16 x 10-19 C. Thus the work function has been calculated without having to know the threshold frequency. To calculate wavelength use the formula wavelength speed divided by frequency.
We have Φ h ν o hcλ o-34 x 3 x 10 8 4800 x 10-10 4144 x 10-19 J-19 16 x 10-19 259 eV. If stopping voltage is given use the equation KEVe to solve for the kinetic energy. In one experiment the electron speed was twice the speed of tha.
Find maximum velocity of photoelectrons. The symbolic representation of the formula given above can be seen as. In mathematical terms phihf-K where phi is the work function and K is the kinetic energy of the electron.
But I cant quite figure out how to calculate the wavelength. The wavelength formula is given by. My guess would be to calculate the energy of the 230 nm photon and then.
This type of problem is good practice at rearranging equations using correct units and tracking significant figures. Work function of silver Φ. It is more usual to work in terms of the angular frequency ω 2πf and wave number k 2πλ so that the de Broglie relations become ω E.
The wavelength can be determined using the frequency and wave speed. Recall that the formula for work function given incident photon wavelength is 𝑊 ℎ 𝑐 𝜆 𝐸. Identify the stopping voltage photon energy and work function of the experiment.
By dividing equation 2 by 3 then we get. It also explains how to calcula. Mentum p a wave of frequency f and wavelength λ given by the de Broglie relations Eq.
It also explains how to use the work function of metals to calculate the threshol. Wavelength of radiation λ. The frequency refers to the number of wave cycles per second.
C f λ. The higher the frequency the lower the wavelength and vice versa. If the work function of the photo emitter is 4 eV find the wavelengths of radiation.
We can use this relationship to calculate the wavelength or frequency of any electromagnetic wave if. To find the work function of the metal we can substitute the graphs horizontal intercept value into this equation. Remember to use the correct units when youre using the formula and writing your answer.
Wavelength Speed of the waveFrequency of the wave As mentioned above all the quantities are represented by a symbol. Frequency f Wavelength λ Speed of lightc f λ c. Y waveAmplitude Mathsin x angleCoef horizontalShift I have to also display the wavelength on the screen.
A metal received light with wavelength 300mathrmnm in experiment A and light with wavelength 500mathrmnm in experiment B. In terms of wavelength to frequency the formula is given as. Find the number of photons striking the metal plate per square metre per second.
Got dE4425 kjmol or 734e-19 J. This is the value of threshold wavelength when the work function of the metal 2 ω 0. The user is able to change the values of the variables waveAmplitude angleCoef and horizontalShift.
Just plug in the waves speed and frequency to solve for the wavelength. Remember that the work function of a metal is the energy required to make an electron free from the surface. This chemistry video tutorial explains how to calculate the energy of a photon given the frequency and the wavelength in nm.
Speed Frequency x Wavelength. This chemistry video tutorial explains how the photoelectric effect works. The photoelectric work function of the metal is 259 eV.
The light falls normally on the plate. Threshold wavelength λ o 4800 Å 4800 x 10-10 m speed of light c 3 x 10 8 ms Plancks constant h 663 x 10-34 Js. This example problem demonstrates how to find the energy of a photon from its wavelengthTo do this you need to use the wave equation to relate wavelength to frequency and Plancks equation to find the energy.
So the correct answer is option C. Just slightly lower in energy than what. Hcwavelength workfunction KE of Energy of electron Now workfunction is given convert it to J Then the velocity of electron is given so find out the KE of electron using 05mv2 in kgm2s2 unit Then plug in back everything in the first equation to get the wavelength of radiation in meter unit.
The wavelength to frequency formula is given by. The work function phi is the amount of energy required to free the electron from the pull of the nuclei of the atoms of the photosurface. 31 With this in mind and making use of what we already know about what the mathematical form is.
H c λ h c λ ω 0 2 ω 0.
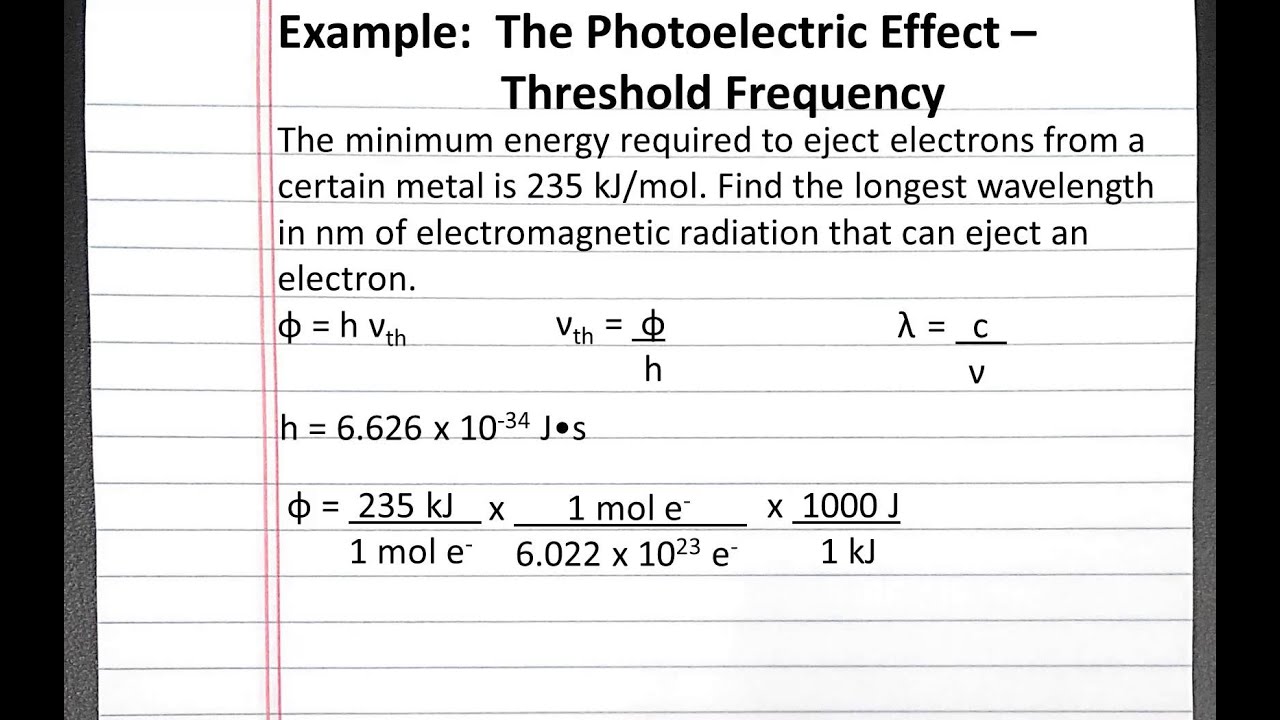
Chemistry 101 Photoelectric Effect Threshold Frequency Youtube
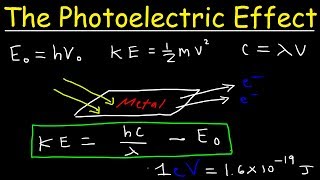
Photoelectric Effect Work Function Threshold Frequency Wavelength Speed Kinetic Energy Electr Youtube
What Will Be The Threshold Wavelength For Potassium If Its Work Function Is 2 Ev Quora
No comments for "How to Find Work Function Given Wavelength"
Post a Comment